For example in our main script, we have these 3 test scenarios
function Test()
{
Scenario1()
Scenario2()
Scenario3()
}
Now, what we want to do is have a hierarchical report:
- The report we want should be high level details first i.e
- TestName Results
- Scenario1 PASS
- Scenario2 PASS
- Scenario3 FAIL
- Then upon click on the + sign, all the low level details (such as steps, images) are present.
An example of how to extend the reporting is provided with Rapise sample test called UsingReporting, if you go to the Start Page in Rapise and click UsingReporting link in the Samples section then you will see a fully created example with embedded comments that explains how to do this.
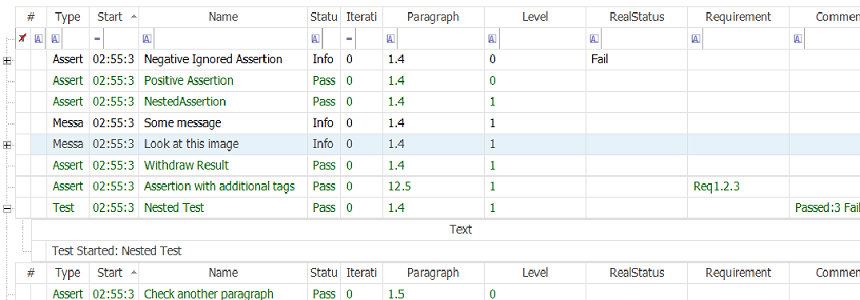
In this example, it generates a hierarchical multi-level report with custom columns using:
Tester.BeginTest("Nested Test");
// ...
Tester.EndTest();
This sample also demonstrates how to use a:
Tester.IgnoreStatus(true);
// ...
Tester.IgnoreStatus(false);
block that forces Rapise to ignore failures within some sequence of actions.
Together these allow you to have much more control over the reporting, and have the report include/exclude certain elements as well as display the data in a hierarchical manner.